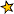As of 2010, there are 118 known elements (in this context, "known" means observed well enough, even from just a few decay products, to have been differentiated from any other element). Of these 118 elements, 94 occur naturally on Earth. Six of these occur in extreme trace quantities: technetium, atomic number 43; promethium, number 61; astatine, number 85; francium, number 87; neptunium, number 93; and plutonium, number 94. These 94 elements, and also possibly element 98 californium, have been detected in the universe at large, in the spectra of stars and also supernovae, where short-lived radioactive elements are newly being made.
The remaining 22 elements, not found on Earth or in astronomical spectra, have been derived artificially.
 https://en.wikipedia.org/wiki/Chemical_element
https://en.wikipedia.org/wiki/Chemical_element
Even though there are 92 elements that are naturally found, only eight of them are common in the rocks that make up the Earth’s outer layer, the crust. Together, these 8 elements make up more than 98% of the crust.
The 8 most common elements in Earth’s crust (by mass):
46.6% Oxygen (O)
27.7% Silicon (Si)
8.1% Aluminum (Al)
5.0% Iron (Fe)
3.6% Calcium (Ca)
2.8% Sodium (Na)
2.6% Potassium (K)
2.1% Magnesium (Mg)
 https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth%27s_crust
https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth%27s_crust