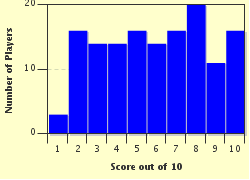
Quiz Answer Key and Fun Facts
1. This compound donates a proton. Therefore...
2. This element has six protons in its nucleus. Therefore...
3. This atom gains an electron. Therefore...
4. This element has a full outer shell of electrons. This may tempt someone to state that "this element is a noble gas". However, which second requirement must be fulfilled for this statement to be true?
5. Atom X shares a pair of electrons with Atom Y. Therefore...
6. This compound has an unpaired electron. Therefore...
7. This compound is an alkene. Therefore...
8. This is a sample of pure helium and it contains Avogadro's number of atoms. Therefore...
9. Atoms in compound X interact strongly with one another, meaning compound X cannot flow or fill the container which it is in. Therefore...
10. Particle X is travelling at Momentum Y in solution. If we calculate Momentum Y, can we know the exact position of particle X?
Source: Author
doublemm
This quiz was reviewed by FunTrivia editor
rossian before going online.
Any errors found in FunTrivia content are routinely corrected through our feedback system.