27. As alkanes are a homologous series, what is the general formula for a linear alkane?
From Quiz Alkanes
Answer:
Cn H2n+2
Wikipedia defines a homologous series as, 'a series of organic compounds with similar chemical properties, members of which differ by a constant relative molecular mass.' For alkanes, the mass of each one differs by 14. This is because of the general formula, Cn H2n+2. For example, in methane, where n=1, the formula CH4 is produced. In ethane, where n=2, the formula C2H6 is found.
Cn Hn exists for benzene, C6H6 but not for a homologous series.
Cn H2n exists for the homologous series of alkenes.
Cn H2n-2 exists for the homologous series of alkynes.
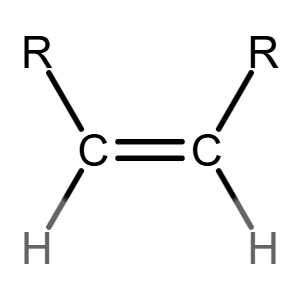 Many people can make little or no sense of diagrams of organic molecules. But even the most complicated molecules feature recognisable "functional groups", configurations of atoms that have specific chemical properties. See how many you can recognise.
Many people can make little or no sense of diagrams of organic molecules. But even the most complicated molecules feature recognisable "functional groups", configurations of atoms that have specific chemical properties. See how many you can recognise. 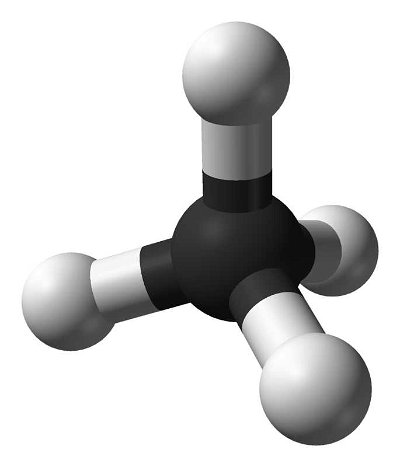
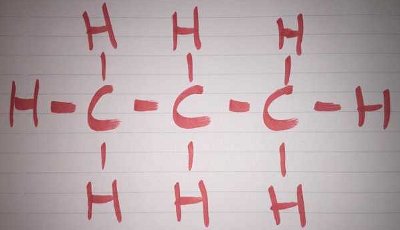 The words 'organic chemistry' may send a chill down the undergraduate spine but you needn't be an expert to have a go at this quiz. Use the photos to discover some of the common functional groups that make up the organic chemist's bread and butter.
The words 'organic chemistry' may send a chill down the undergraduate spine but you needn't be an expert to have a go at this quiz. Use the photos to discover some of the common functional groups that make up the organic chemist's bread and butter.  Quick Question
Quick Question = Top 5% Rated Quiz,
= Top 5% Rated Quiz,
 Top 10% Rated Quiz,
Top 10% Rated Quiz,
 Top 20% Rated Quiz,
Top 20% Rated Quiz,
 A Well Rated Quiz
A Well Rated Quiz