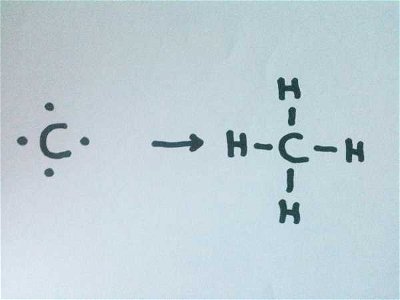
14. By telling you that you contain 22 protons in total, you should gather that you are a relatively small compound. If I were also to tell you that you are uncharged, what could you tell me about the number of electrons you contain?
From Quiz Detective Chemistry
Answer:
You have 22
Atoms are made up of protons (positively charged particles), electrons (negatively charged particles), and neutrons (uncharged particles). For ease of understanding, we say that one proton has a charge of +1 and an electron has a charge of -1. Therefore, in an uncharged compound, the electron number would have to balance the proton number.
Electrons can be lost or gained by atoms/compounds, thus providing them with a charge. An example of this is in table salt. Chlorine (Cl2) reacts with 2 sodium atoms, each Cl atom in Cl2 gains one electron from a sodium atom. The result are negatively charged chloride ions and positively charged sodium ions which join together to form the ionic compound, sodium chloride (salt).










 Quick Question
Quick Question