Naming Molecules Trivia Quiz
An introduction to systematic nomenclature
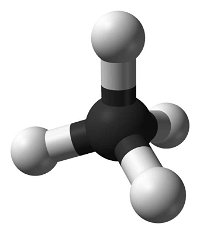
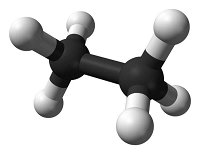
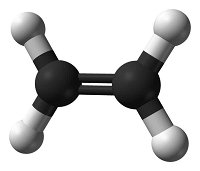
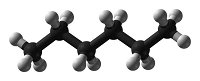
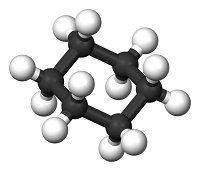
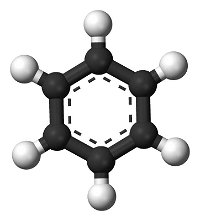
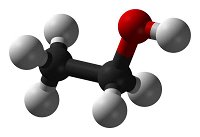
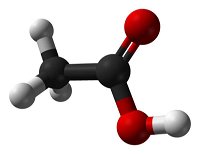
Systematic nomenclature? This means using names for chemicals that describe what is in them. Salt is sodium chloride, for example. Match each diagram of a molecule with the name and molecular formula of the chemical it represents.
by looney_tunes.
Estimated time: 3 mins.