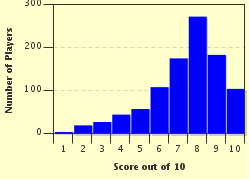
Quiz Answer Key and Fun Facts
1. Alkanes are hydrocarbons, as they only contain the elements hydrogen and carbon. What is the name of the simplest alkane?
2. As alkanes are a homologous series, what is the general formula for a linear alkane?
3. Carbon is capable of catenation, which is the ability of an element to form covalent bonds to itself. Carbon can form single, double or triple bonds to other carbon atoms, but which of these is present in alkanes?
4. How many carbon atoms does heptane contain?
5. What is formed when an alkane undergoes complete combustion?
6. In general terms, what is the name given to the product when an alkane reacts with a halogen?
7. How many carbon atoms must be present in an alkane for the molecule to show structural isomerism?
8. What is the general term used to describe an alkane in a ring structure?
9. What is the trend in boiling points for straight chain alkanes?
10. When an alkane exists in a ring structure, it has a higher boiling point than the corresponding straight chain alkane.
Source: Author
Milky1989
This quiz was reviewed by FunTrivia editor
crisw before going online.
Any errors found in FunTrivia content are routinely corrected through our feedback system.